Published 13:13 IST, May 28th 2021
DRDO anti-COVID drug '2DG' to be available at discounted rates; See price & availability
The Defense Research & Development Organisation (DRDO) had released its anti-COVID-19 drug '2DG' on May 17, it was developed in collab with Dr Reddy's Lab.
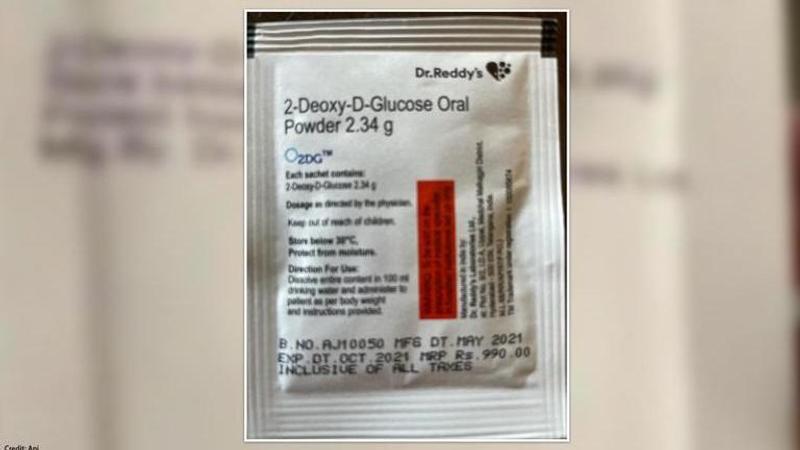
The Defense Research & Development Organisation (DRDO) announced that it had released its 2DG anti-COVID 19 drug on May 17. In another development, it was announced that the drug has been priced at Rs 990 per sachet by Dr Reddy’s lab. However, government officials have informed that the DRDO anti-COVID drug will be provided at a discounted price for the government hospitals. In addition, the drug price is also discounted for central and state governments.
The first batch of 2DG launched
Defence Minister Rajnath Singh and Union Health Minister Dr Harsh Vardhan had released the first batch of anti-COVID-19 drug D2G that is manufactured by the DRDO on May 17. Dr Harsh Vardhan had asserted that the drug would boost recovery in Coronavirus patients and lead to a reduced dependency on oxygen. The Health Minister also hailed the efforts of scientists at the DRDO. The indigenously developed drug was handed over by Rajnath Singh to Dr Harsh Vardhan who then handed it over to AIIMS Director Dr Randeep Guleria. It was granted emergency approval on May 1.
"Whenever our country was in need. the DRDO came forward to work for the nation. Our experts are saying that there can be another possible wave. We will not be at ease and will not be tired but will keep fighting and will win against COVID-19," said Dr Harsh Vardhan
About DRDO anti-COVID drug 2DG
The drug has been developed by DRDO scientists at the Institute of Nuclear Medicine and Allied Sciences (INMAS). In addition, the drug has also been developed in collaboration with Dr Reddy’s Laboratories (DRL) in Hyderabad. After lab experiments, were conducted in April 2020, the 2-DG was found to be safe and effective against Coronavirus. The Phase-II trials had also indicated that the drug was effective against the virus with signs of recovery among the COVID-19 patients. The drug was tested again in Phase-IIa and Phase-IIb trials at six and 11 hospitals respectively on 110 patients. In addition, the DRDO had also received approval by the DGCI to conduct Phase-III trials which were held between December 2020-March 2021 at 27 hospitals across India on 220 patients. Results concluded that patients recover faster along with a significant decline in the use of oxygen.
Updated 13:47 IST, May 28th 2021




