Published 11:48 IST, February 9th 2022
FabiSpray: Glenmark launches COVID nasal spray in India for adults; check details
Glenmark Pharmaceuticals Limited (Glenmark) has launched Nitric Oxide Nasal Spray, under the brand name FabiSpray for the treatment of adult COVID patients.
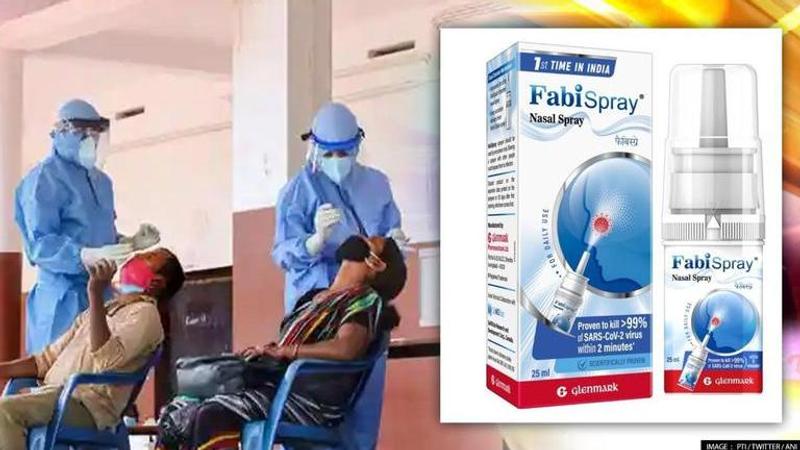
In a major boost to India's fight against COVID-19, Glenmark Pharmaceuticals Limited (Glenmark) has launched Nitric Oxide Nasal Spray, under the brand name FabiSpray for the treatment of adult patients who have contracted SARS-CoV-2 and who have are at higher risk of progressing the infection.
In collaboration with Canada's SaNOtize Research & Development Corporation, the Indian entity is set to manufacture and market the same after it received a nod from the Drug Controller General of India (DGCI).
What is FabiSpray?
Formulated to fight the SARS-CoV-2 virus while it prevails in the upper tracts, FabiSpray has proven to contain anti-microbial properties with a direct effect on the virus, leading to its destruction. The usage noted that the Nitric Oxide Nasal Spray (NONS), upon being sprayed over nasal mucosa, acts as a physical and chemical barrier to the virus, thus preventing its entry and incubation into one's lungs.
FabiSpray's availability
As a part of the exclusive long term strategic partnership, Glenmark and SaNOtize will manufacture, market, and distribute its Nitric Oxide Nasal Spray (NONS) in India, Singapore, Malaysia, Hong Kong, Taiwan, Nepal, Brunei, Cambodia, Laos, Myanmar, Sri Lanka, Timor Leste, and Vietnam, according to a statement by the company.
FabiSpray's price
While the market price of Glenmark's COVID-19 nasal spray is unknown so far, Glenmark Pharmaceutical's share price rose 2% on the morning of February 9, right after the product was launched for Indian patients. The entity was quoting at Rs 494.95 on the Bombay Stock Exchange, the shared projected an up Rs 9.90 or 2.04% at the BSE.
Glenmark launches COVID nasal spray in collaboration with SaNOtize
In March 2021, SaNOtize’s clinical trials showed NONS was a safe and effective antiviral treatment to prevent the transmission of COVID‐19, shorten its course, and reduce the severity of symptoms.
In the first 24 hours, NONS reduced the average viral load by around 95%, and then by more than 99% within 72 hours. It has been tested in healthy volunteers and patients as a part of UK and Canada clinical trials, the company added.
In early July 2021, Glenmark presented a proposal to the subject expert committee of the Central Drugs Standard Control Organisation (CDSCO) for emergency approval of the import and marketing of the nasal spray.
"NONS is one of the few novel therapeutic treatments, outside of expensive monoclonal antibodies, that is proven to reduce SARS‐CoV‐2 viral load in humans. NONS has already received a CE (conformite' europeenne) mark in Europe, which is an equivalent of marketing authorization in case of a Medical Device (CE mark confirms that the medical device meets certain 'essential requirements' of the European General Medical Devices Directive and is safe for the intended purpose),'' Glenmark's statement read.
Updated 11:48 IST, February 9th 2022




