Published 18:35 IST, April 24th 2020
Human trial of COVID-19 vaccine starts in UK: Here's how it works
The vaccine has been developed to protect healthy people from COVID-19 and it will be injected into 400 individuals out of 800 who have volunteered for it.
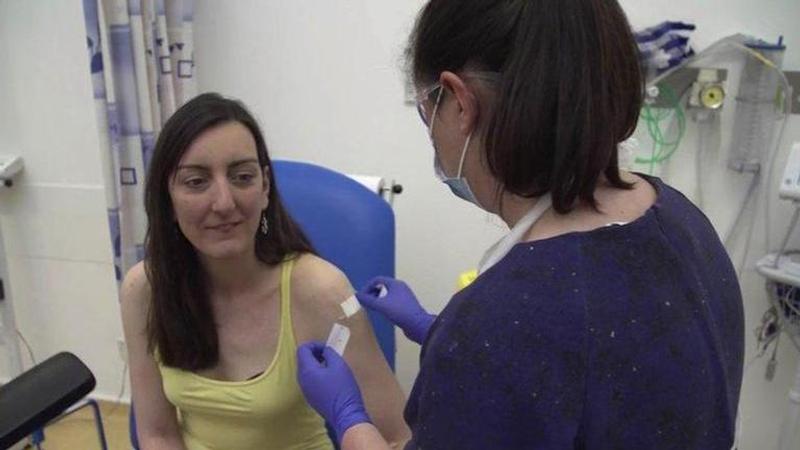
As several countries race to develop vaccines to combat the novel coronavirus, the United Kingdom started its first human trial on April 23. Two volunteers were injected with the potential COVID-19 vaccine developed by Oxford University and all eyes will be on the human trial which could prove a major breakthrough in the fight against the virus.
The vaccine has been developed to protect healthy people from COVID-19 and it will be injected into 400 individuals out of 800 who have volunteered for the study. The vaccine is made of a weakened and modified version of adenovirus (a common cold virus) from chimpanzees. The team of researchers extracted the genes for the spike protein on the surface of the coronavirus.
When the vaccine is injected into a body, the vaccine enters cells and start producing coronavirus spike protein. The spike protein prompts the immune system to produce antibodies and trigger T-cells to destroy the infected cells. If the individual encounters the novel coronavirus, the antibodies and T-cells are activated to fight the virus.
"This vaccine aims to turn the virus' most potent weapon, its spikes, against it - raising antibodies that stick to them allowing the immune system to lock onto and destroy the virus," Saul Faust, the director of the National Institute for Health Research's Southampton Clinical Research Facility at the University Hospital Southampton, said in the statement.
Confident about efficacy
Sarah Gilbert, the lead researcher of the vaccine development programme, had told a virtual conference on April 17 that her team is confident about the efficacy of ChAdOx1 vaccine. The team had already been working on a plan for an unknown disease, named Disease X, which would have caused a pandemic.
Gilbert said that 12 clinical trials have already been conducted against different diseases by using ChAdOx1 technology and it has shown strong immune response with a single dose. The Oxford vaccine group has claimed that it will able to provide one million doses of the vaccine by September 2019.
(Image credit: AP)
Updated 18:35 IST, April 24th 2020




