Published 08:32 IST, August 9th 2020
UK: Nearly 750,000 COVID-19 test kits recalled due to sterility issues
UK’s medicines and healthcare products regulator (MHRA) has asked Randox to recall the test kits sent out to care homes and individuals owing to safety concerns
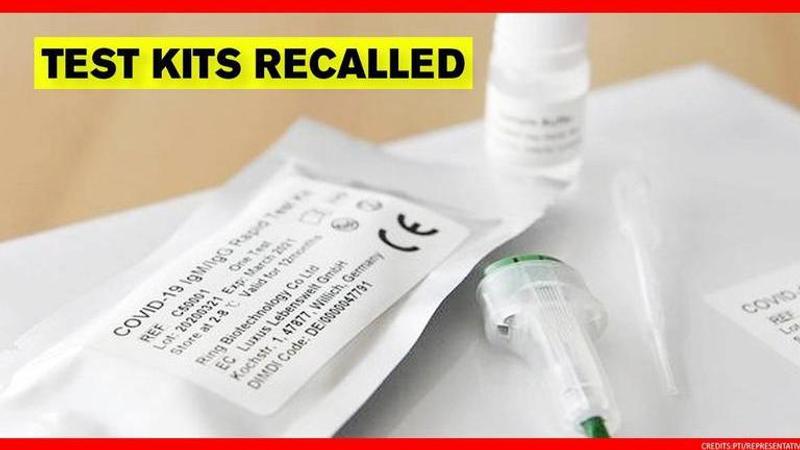
The United Kingdom government has recalled nearly 750,000 unused COVID-19 testing kits due to safety concerns. According to international media reports, UK’s medicines and healthcare products regulator (MHRA) asked Randox to recall the kits sent out to care homes and individuals. The government is reported to have stated it as a ‘precautionary measure’ and said the risk to safety was low.
After recalling the COVID-19 testing kits, the UK Health Secretary Matt Hancock reportedly said the Randox kits should not be used until further notice. Back in July, Hancock had said that the swabs in the testing kits were ‘not up to standard’. The UK government then had instructed care homes and members of the public to immediately stop using Randox kits because of sterility issues.
As per reports, the UK Department for Health and Social care said that results from Randox tests were unaffected. A spokesperson informed that the government has high safety standards for the COVID-19 tests while adding that following the order, Randox has now recalled all tests kits.
Randox to recall all kits from NHS
According to reports, the DHSC is now in the process of contacting every setting which has sent test kits for processing to remind them of the instruction to ensure kits are not used, though the regulatory responsibility to issue a recall lies with Randox. The department has reportedly also instructed Randox to recall all test kits from the NHS test and trace testing settings.
Meanwhile, the Randox Laboratories spokesperson is reported to have said that it had not been provided with satisfactory evidence to support the CE marking of COVID-19 sample collection kits purchased from an external supplier. They further noted that CE certification by the supplier and that some kits remain in the field at this time. However, Randox has, as a regulatory measure, initiated the recall of those kits used within the national testing programme.
Randox further added that will continue to provide high-volume COVID-19 testing to the national testing programme from their laboratories, based on sample collection kits from other providers.
(Image: Rep/AP)
Updated 08:32 IST, August 9th 2020




