Published 22:53 IST, August 6th 2020
UK COVID-19 vaccine trial participants on immunizing safety
Scientists at Imperial College London say they are immunizing hundreds of people with an experimental coronavirus vaccine in an early trial after seeing no worrying safety problems in those vaccinated so far.
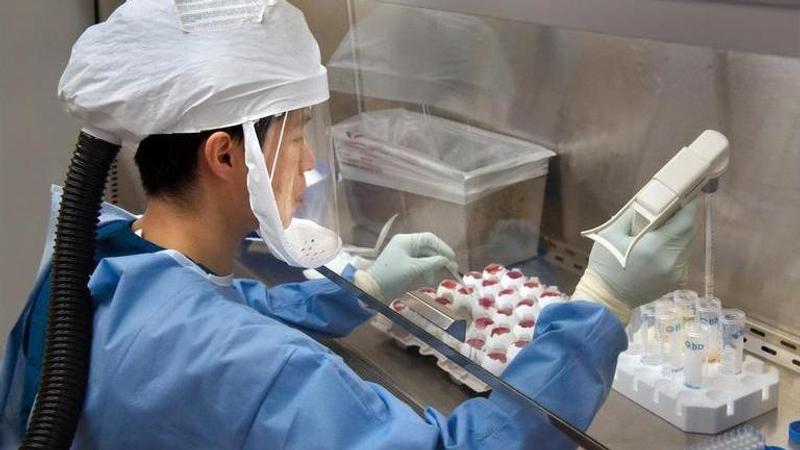
Scientists at Imperial College London say they are immunizing hundreds of people with an experimental coronavirus vaccine in an early trial after seeing no worrying safety problems in those vaccinated so far.
One of the trial participants, Philip, told the Associated Press that he was feeling fine as he received the second dose of the vaccine. "There's a small group of people at the start who receive the vaccine to be checked for any reactions, effects, but as far as I'm aware everything's going ok, it's fine."
The Imperial vaccine uses synthetic strands of genetic code based on the virus.
Once injected into the muscle, the body's own cells are instructed to make copies of a spiky protein on the coronavirus.
That should in turn trigger an immune response so the body can fight off any future COVID-19 infection.
Trial participant Philip said taking part in the trial and knowing he was receiving the vaccine – not just a placebo - hadn't changed his approach to everyday life.
"Even now it's just, carry on as normal as best you can and there's no difference to how I behaved last time, apart from masks, washing your hands, forming queues, more queues than normal," he said.
Last week, the world's biggest coronavirus vaccine study started in the US, with the first of 30,000 planned volunteers getting immunized by shots created by the US National Institutes of Health and Moderna Inc.
Several other vaccines made by China and by Britain's Oxford University, based on different vaccine technologies, began smaller final-stage tests in Brazil and other hard-hit countries earlier this month.
The World Health Organization has said multiple vaccine approaches are necessary for COVID-19, noting that the usual success rate for vaccine development is about 10%.
"This is a time of unprecedented vaccine research across the globe," said Doctor Katrina Pollock, Senior clinical research fellow in vaccinology and chief investigator on the Imperial College vaccine trials.
"I think it's likely we're going to need more than one vaccine. We may need different types of vaccines for different ages for example, the obvious paradigm for that would be the flu vaccine, we'd give different vaccines for different ages in this country and you know, it's a rapidly evolving situation. So it's one we're all keeping our eye on and it's great to be involved in this research," she added.
(Image: Unsplash)
Updated 22:53 IST, August 6th 2020




